eCTD in Ukraine: A New Format for Registration Dossiers and Practical Aspects of Implementation
On August 18, a landmark event took place in the regulatory sphere: Ukraine officially transitioned to the electronic Common Technical Document (eCTD) format. From this date forward, registration dossiers for new marketing authorizations must be submitted exclusively in electronic format, with the exception of procedures regulated by Orders of the Ministry of Health of Ukraine No. 1245 dated November 17, 2016, and No. 1391 dated June 15, 2020. This regulatory shift brings Ukrainian legislation closer to European Union practices and fundamentally changes the day-to-day operations of regulatory affairs specialists.
The transition to electronic format is not just about compliance with new requirements — it also significantly accelerates and increases the efficiency of pharmaceutical companies’ regulatory departments. There is no longer a need to prepare cover pages and tables of contents, print and copy multi-volume paper dossiers, sign and stamp documents by hand, paginate materials, create physical “tabs,” or personally visit the State Expert Center (SMDC) to submit dossiers or respond to deficiency letters. All these processes have now been digitalized: the dossier is compiled using specialized software, signed with a qualified electronic signature (QES), validated with a software validator, and submitted via archive upload in the Applicant’s Portal. This approach enables remote workflows, simplifies version control, allows for quick keyword search, supports automated generation of certain dossier sections, and ensures proper formatting.
According to our estimates, the transition to eCTD can save 3 to 7 months of work annually per regulatory affairs manager.This means a substantial portion of routine work is eliminated, allowing specialists to focus on high-value regulatory tasks, thus improving both the quality and speed of their work.
To use eCTD effectively, one needs not only basic theoretical knowledge, but also appropriate software tools and hands-on experience. The core structure of the new format remains the familiar CTD layout, approved by Order of the Ministry of Health of Ukraine No. 426 dated August 26, 2005, which has not fundamentally changed. However, new attributes and technical specifications have been added, primarily within Module 1. These are defined by the Ministry of Health Order No. 691 dated April 23, 2024, along with subsequent amendments. In addition, Europe has accumulated substantial experience in eCTD implementation since its adoption in 2003. The EU has developed numerous guidelines, clarifications, and best practices, many of which are available at esubmission.ema.europa.eu/ectd, and some of these are now being adapted for use in Ukraine. While Ukraine is aligning its regulatory requirements with those of the EU, an EU-format registration dossier cannot be submitted in Ukraine “as-is” — it must undergo localization and adaptation to comply with Ukrainian national standards.
The eCTD lifecycle in any country begins with the assignment of a universally unique identifier (UUID). In Ukraine, a new UUID must be generated for each registration dossier. This UUID is linked to a specific medicinal product and remains permanently assigned to that dossier throughout its entire lifecycle. All future submissions related to that product — such as variations, renewals, or responses to deficiency letters — must use this same UUID. This mechanism ensures consistent traceability, simplifies lifecycle management, and aligns with international standards for regulatory submissions.
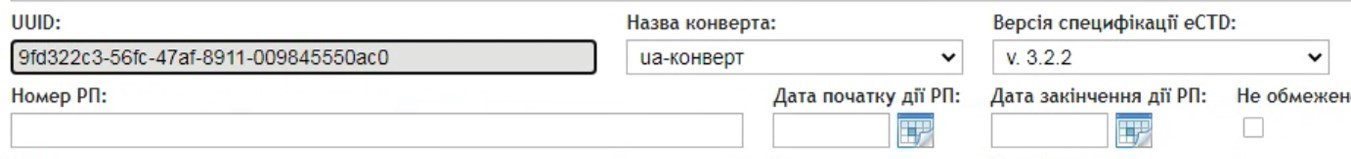
UUID: A Constant Identifier Throughout the Entire Dossier Lifecycle
In Ukraine, every initial submission of a registration dossier in eCTD format begins with sequence “0000”, regardless of the type of procedure — whether it is a new marketing authorization, renewal, variation, or baseline submission. The eCTD-RIMS system developed by IRIS-Soft includes a built-in UUID generator that automatically creates a new UUID for the initial sequence “0000”. The system also allows for the import of an existing UUID, which ensures continuity of the dossier lifecycle, for example, in the case of a change of applicant.
Each subsequent submission increments the sequence number by one: the response to the first round of comments will carry sequence “0001”, the second — “0002”, and so on. If a medicinal product is already registered in another country (e.g., within the EU) and has an existing regulatory history, the sequence numbering will not align between markets. Therefore, sequence tracking must be managed separately for each jurisdiction. For instance, the same variation may be submitted as sequence “0045” in Germany, while it would be “0004” in Ukraine.
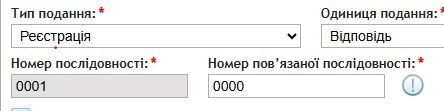
When submitting responses to deficiency letters, it is mandatory to indicate the related sequence, i.e., the submission to which the response pertains. For example, the second response to a deficiency letter during a new registration procedure would be submitted as sequence “0002”, with the related sequence marked as “0000”.
All submissions form part of the dossier’s lifecycle history, which is presented in the form of a tracking table. This table is attached to the cover letter and provides a concise summary of each submission along with the corresponding sequence numbers. The tracking table serves as a transparent monitoring tool for all regulatory actions associated with the dossier, ensuring clear documentation and traceability throughout the product’s lifecycle.
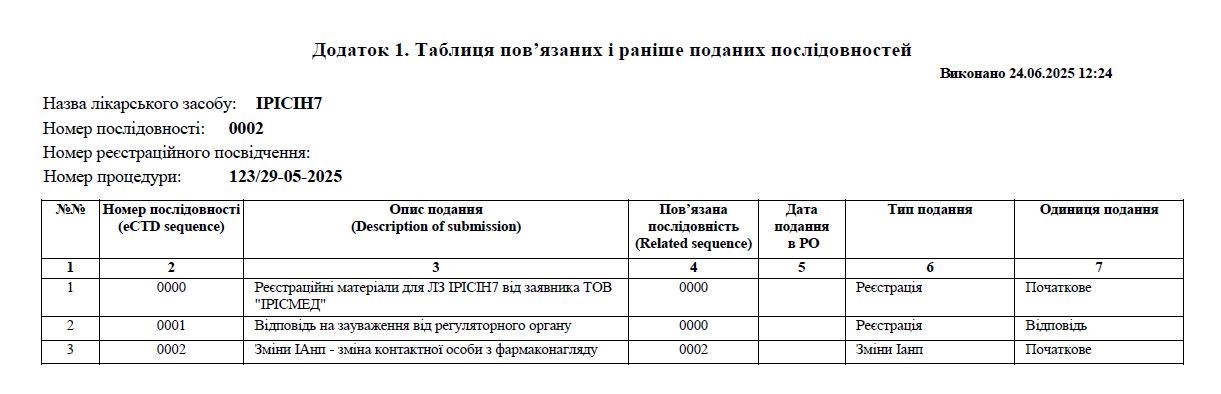
To prepare a registration dossier in eCTD format, it is essential to use dedicated software, which becomes the core tool in the daily work of regulatory affairs professionals. This software must be user-friendly, adapted to Ukrainian regulatory requirements, and its developer should provide comprehensive training and technical support, preferably in the Ukrainian language.
The dossier itself consists of multiple PDF files, each corresponding to a specific section of the Common Technical Document. A single section may include several documents — for example, section 3.2.S may contain materials from different manufacturers of active pharmaceutical ingredients (APIs). All PDF files — except for certain administrative documents in Module 1 — must be size-optimized and OCR-processed (optical character recognition) to enable text-based searching. This ensures regulatory reviewers can easily navigate, search, and assess dossier content in a structured and efficient way.
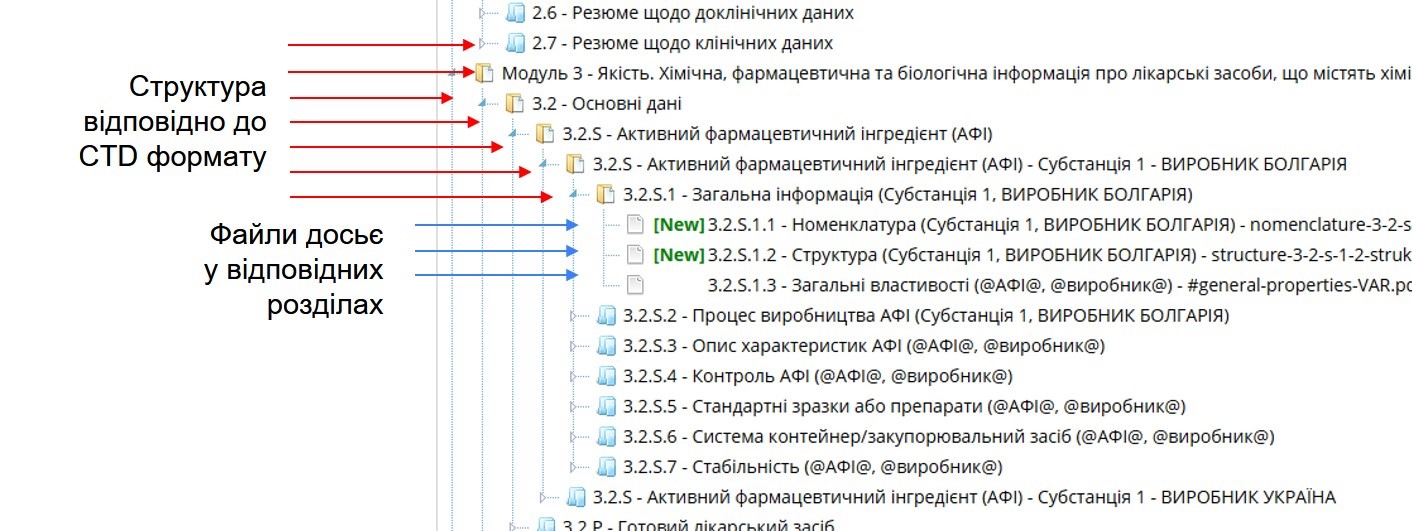
Sample eCTD Dossier Structure with Two API Manufacturers
Certain parts of the registration dossier in eCTD format — for example, quality control methods, the Summary of Product Characteristics (SmPC), labelling, and the Risk Management Plan (RMP), along with any subsequent amendments — must be submitted in PDF format as part of the main eCTD sequence. In addition, these documents must also be provided in editable format (.docx) within a separate folder titled “Working Documents”.

Together with the dossier, a cover letter must be submitted, which includes a description of the regulatory action, related sequences, the tracking table mentioned above, a list of documents, and information on the number of files in the submission.
Administrative documents in sections 1.0 and 1.2 (the cover letter, letters of guarantee, Annexes 29 and 30, the registration form with annexes, CoPP, licenses, confirmation of compliance with Good Manufacturing Practice (GMP) requirements, and others) must be signed with a Qualified Electronic Signature (QES) using keys issued by accredited certification authorities in accordance with Ukrainian legislation. Such signatures are applied to PDF files in PAdES format, which preserves the structure of the files and prevents any changes to their content. The eCTD-RIMS system includes integrated electronic signature libraries, which are user-friendly and allow documents to be signed directly within the system.
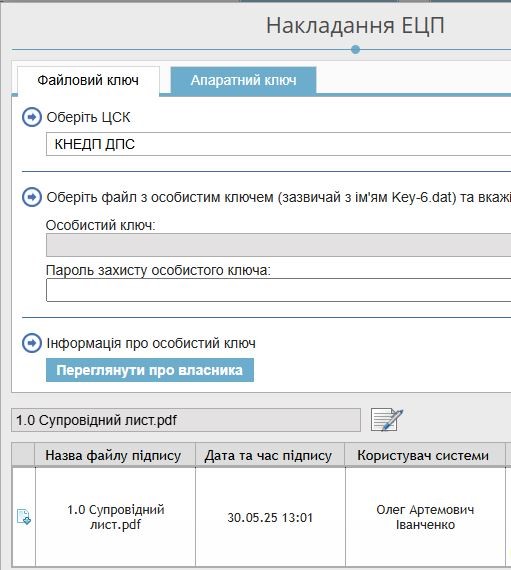
Before finalizing the dossier, a mandatory stage is validation, i.e. checking the files for compliance with technical requirements. It is carried out in the eCTD-RIMS system itself and covers the correct organization of the eCTD structure (modules, subfolders, XML-backbone), compliance of file formats (searchable pdf with bookmarks without encryption), availability and correctness of metadata (product name, procedure type, sequence number, UUID, applicant data), as well as compliance with the life cycle rules using the attributes “new”, “replace”, “append” or “delete”. Additionally, compliance with the requirements for Module 1 defined by the Ukrainian specification is checked.
When the dossier is formed, signed and successfully validated by the applicant, it is published. Publishing is a technical process during which a “main” main XML file is formed, all materials are packed into an archive, and this archive is stored on the computer of the registration specialist. This archive is then uploaded to the Applicant’s Portal and is considered the official submission to the State Expert Center of the Ministry of Health of Ukraine (SMDC). After being uploaded via the Applicant’s Portal, the eCTD dossier undergoes validation on the side of the SMDC. If any errors are detected, the materials are not accepted and require correction and resubmission.
The eCTD-RIMS software, developed by IRIS-Soft, fully complies with the requirements of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the national specification for Module 1, approved by Order of the Ministry of Health of Ukraine No. 691 dated April 23, 2024, as amended. The system is already used by a large number of applicants for the preparation and submission of dossiers to the State Expert Center of the Ministry of Health of Ukraine. In addition to the basic functions necessary for working with eCTD, it includes a number of additional features that simplify the work of a registration specialist: a UUID generator, automatic renaming of files from Cyrillic to Latin, a separate folder for “Working documents”, built-in qualified electronic signature (QES) functionality, validator, rapid digitization module, file repository. The system integrates with other solutions, in particular with the DSBase pharmacovigilance platform. IRIS-Soft provides user training and prompt support in the Ukrainian language, which is a significant advantage in day-to-day work.
We invite you to a free online training session dedicated to the implementation of eCTD in Ukraine will take place. The seminar program includes both theoretical and practical components. In the first part, participants will receive a structured explanation of the requirements and specific aspects of submitting dossiers in eCTD format. In the second part, we will demonstrate the use of the eCTD-RIMS software, provide a detailed explanation of the main steps for preparing materials for new marketing authorization applications, renewals, variations, and baseline submissions, and review other key aspects of working with the electronic format. To participate in the training, you must register.
Other News
Сompletion of the development of the new Digital Medical Device Quality System eMDD-RIMS
We announce the completion of the development of the new Digital Medical Device Quality System eMDD-RIMS. eMDD-RIMS is a document management system for manufacturers, authorized representatives and distributors of medical devices that significantly simplifies the management of regulatory and quality documentation. The system allows you to create a centralized documentation repository with access for all […]
iRIS Soft Participates in the “PHARMEXPERT 2025” Forum
The iRIS Soft team actively participated in the “PHARMEXPERT 2025: eCTD and Pricing in Ukraine — A Strategic Path to Integration into the International Regulatory Environment” forum, held on June 17, 2025, in Kyiv. During the event, the company presented its own innovative solution — the Electronic Dossier Management System eCTD-RIMS. iRIS Soft representatives joined […]