Germes – automated literature screening

The platform enables search planning by trade name or international nonproprietary name, regular monitoring of periodicals and online resources, downloading full-text publications and hyperlinks with subsequent storage in the database, logging search results, and generating reports on monitoring outcomes.
The computerized system provides automatic internet searches for safety and efficacy data on medicinal products based on:
- Keywords;
- Specified websites;
- A defined time period.
The results of searches and literature analysis are used for the preparation of registration dossiers, periodic safety reports, and other pharmacovigilance-related documents.
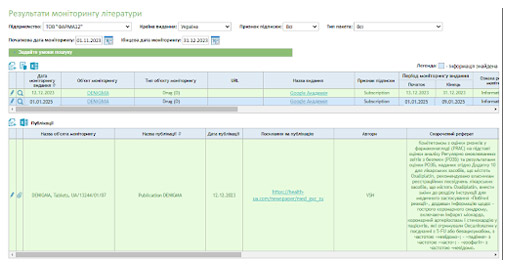
The Germes PV system by iRIS Soft is an effective tool for supporting pharmacovigilance and ensuring regulatory compliance. Germes can be provided as a standalone product or as part of the DSBase platform.
Key Features of Germes PV:
The system monitors the websites of the following international and national regulatory authorities:
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- U.S. Food and Drug Administration (FDA)
- Medicines and Healthcare Products Regulatory Agency (MHRA, UK)
- Health Canada
- Therapeutic Goods Administration (TGA, Australia)
- Swissmedic (Swiss Agency for Therapeutic Products)
- Pharmaceuticals and Medical Devices Agency (PMDA, Japan)
- PubMed, Trip Medical Database, International Network of Agencies for Health Technology Assessment, and many other electronic libraries
- Websites of regulatory authorities in the EU, USA, Asia, EAEU, and other regions