eMDD RIMS – digital regulatory tool for medical devices

eMDD RIMS by iRIS Soft is a tool for digitizing the quality management system in compliance with ISO 13485, collecting and processing data on medical device incidents, and managing regulatory documentation for manufacturers, Authorized Representatives, and distributors of medical devices. The system allows users to:
- Digitize documents, quality management systems, and process management
- Improve compliance with ISO 13485 and other standards
- Ensure consistency of information across documents
- Accelerate and simplify labeling processes
- Automate workflows, reducing risks and costs
- Enable centralized document storage, assign roles, and provide shared access
- Ensure transparency and control at every stage, reducing time for approval and validation
- Prevent errors associated with manual document handling
The system fully complies with electronic document management requirements, including support for Qualified Electronic Signatures (QES).
Key Functional Capabilities of eMDD RIMS:
Technological Features
The eMDD RIMS electronic document management system is designed with a focus on flexibility, scalability, and security, enabling efficient support and automation of complex data processing workflows. The system’s key technological advantages include:
Залпове оцифрування документів:
Batch Document Digitization: The initial population of the Electronic Document Management System (EDMS) is performed using batch digitization technology, which processes large volumes of accumulated paper documents. The converted electronic documents are then integrated into the Electronic Document Repository to ensure long-term storage and seamless enterprise-wide utilization.
Modular Architecture: The system is built on a modular architecture, allowing customization and expansion of functionalities to meet the specific needs of each client. This enables adaptation for both small businesses and large corporations, ensuring the necessary level of flexibility.
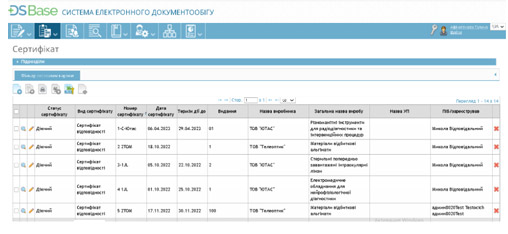
ISO 13485 Compliance at its Core: The primary objective of the system is to implement best practices in quality management documentation for manufacturers and distributors. eMDD RIMS ensures data consistency across different documents (declarations, certificates, labeling, instructions, customs documents, etc.) and includes facility and equipment modules, QR coding, and an electronic document repository. This facilitates validation management, metrological calibration, approvals, and more.
Cloud and On-Premise Deployment: eMDD RIMS offers a choice between cloud-based and on-premise deployment, allowing clients to optimize costs and select the most convenient access method. This also enhances security configuration flexibility and ensures data availability.

Centralized Data Storage: All data is stored in a centralized repository, ensuring fast and controlled access for users. The system also supports automatic backups, enhancing document storage reliability.
Integration Capabilities: The system includes interface support for integration with both internal and external systems. This facilitates seamless implementation into existing client infrastructures and enables efficient data exchange with other information systems.
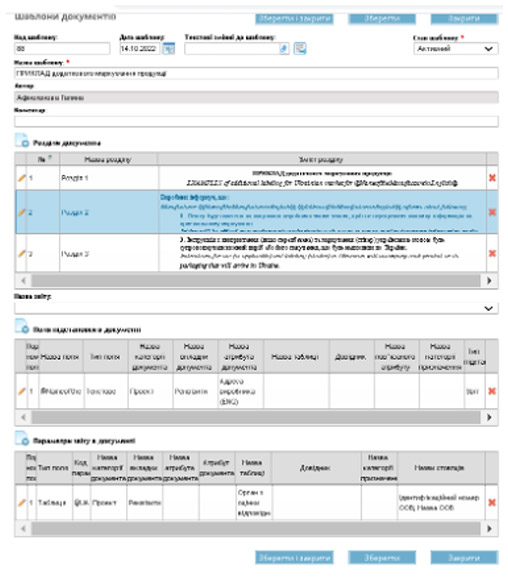
Adaptation to Ukrainian Legislation
One of the core functions of the Enterprise Quality Management System is the development, review, and implementation of various regulatory and accounting documents in compliance with the existing regulatory framework. The system ensures compliance with:
- Technical Regulations on Medical Devices, In Vitro Diagnostic Devices, and Active Implantable Medical Devices, approved by CMU Resolutions No. 753, 754, and 755
- ISO 13485 standard
Key Issues Addressed by the EDMS:
- Development of policies and instructions for working with electronic documents within the enterprise
- Procedures for registration and accounting of electronic documents
- Implementation of electronic signature practices for employees
- Access control to documents for different structural units and personnel
- Monitoring document validity periods and managing updates
- Automated electronic distribution of documents
- Controlled printing of document copies
- Ensuring the removal of outdated document copies from structural units
- Preparation and scanning of paper documents
- Definition of document storage policies and execution of archival expertise