eGMP/GDP – digital quality management of manufacturer or distributor

The eGMP/GDP EDMS by iRIS Soft is a key factor in enterprise digitalization that allows:
- Digital transformation of documents, quality management systems, and business processes
- Enhanced compliance with GMP/GDP standards
- Automation of processes to reduce risks and operational costs
- Centralized document storage, role-based access control, and secure collaboration
- Increased transparency and control at every stage, reducing approval and review times
- Minimization of errors associated with manual document handling
The system includes registry modules for facilities, equipment, and pharmaceutical production/storage systems, as well as tools for task execution monitoring and KPI evaluation. These features enable efficient digital management and planning within the pharmaceutical quality system.
The system complies with all electronic document management requirements, including support for a Qualified Electronic Signature (QES) and compliance with 21 CFR Part 11.
Key аunctional сapabilities of eGMP/GDP:
This is an innovative platform for automating document management, aimed at optimizing business processes, reducing risks, and increasing enterprise productivity. eGMP/GDP by iRIS Soft supports centralized document storage, automation of creation, approval, validation, and lifecycle monitoring of documents. Built-in security tools, including a Qualified Electronic Signature, ensure compliance with regulatory requirements and data protection. The platform is scalable, customizable, and supports mobile access, ensuring convenience for work anytime and anywhere.
Technological аeatures
The eGMP/GDP Electronic Document Management System (EDMS) is designed with a focus on flexibility, scalability, and security, enabling the efficient support and automation of complex data processing workflows. The system’s key technological advantages include:
e-CTD RIMS
Bulk Document Digitization: the initial EDMS population is carried out using digitization technology, allowing for the processing of large volumes of accumulated paper documents. The resulting electronic documents are then incorporated into the Electronic Document Database to ensure guaranteed long-term storage and accessibility within the enterprise.
Modular architecture: the system is built on a modular architecture, enabling customization and functional expansion to meet the specific needs of each client. This approach allows both small businesses and large corporations to adapt the solution while maintaining the necessary level of flexibility.
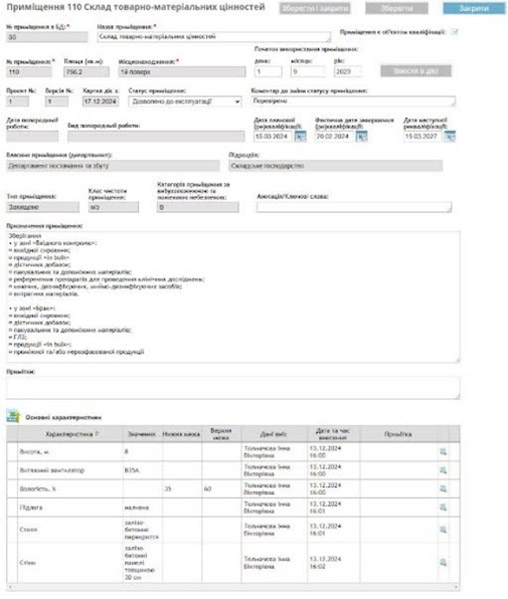
GxP compliance at its core: the primary goal of the EDMS is to implement best practices for document management within Good Practices (GxP) frameworks, including manufacturing and distribution. The system includes modules for facilities and equipment management, QR coding, and a repository for electronic documents, simplifying validation, metrological verification, and access control.
Cloud and on-premises deployment: eGMP/GDP EDMS offers a choice between cloud-based deployment and on-premises server installations, allowing clients to optimize costs and select the most convenient method of accessing the system. This flexibility also enhances data security and availability configurations.
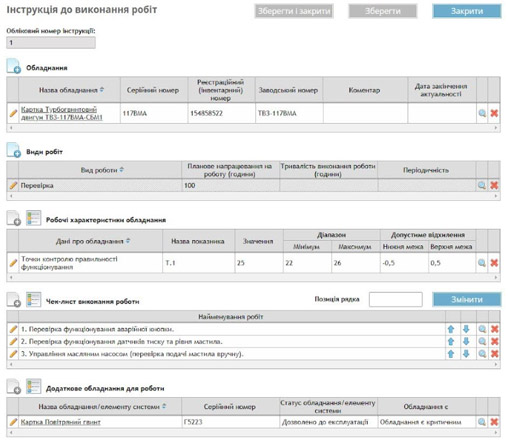
Centralized data repository: all data is stored within a single, centralized repository, ensuring fast and controlled access to information for users. Additionally, the system supports automated backups, providing increased reliability and security for stored documents.
Integration interface support: the system includes integration capabilities with both internal and external systems, facilitating seamless implementation into existing IT infrastructures and streamlining data exchange with other information systems.
Adaptation to Ukrainian legislation
One of the primary functions of the Enterprise Quality Assurance System is the development, review, and implementation of various regulatory and record-keeping documents in compliance with the current regulatory framework. This includes Guideline ST-N MOH 42-4.0:2020 – Medicinal Products. Good Manufacturing Practice (GMP) and Guideline ST-N MOH 42-4.3:2011 – Medicinal Products. Pharmaceutical Quality System (ICH Q10).
Implementation of ALCOA Principles in the eGMP/GDP EDMS by iRIS Soft:
ALCOA is an acronym representing the fundamental requirements for data integrity in the system:
- Attributable: The system records information on who acted and when. This data is automatically logged in each system registration record.
- Legible: Data must remain readable throughout its lifecycle. This is ensured through database backups, and data security measures, including antivirus protection, to prevent data corruption or loss.
- Contemporaneous: Data must be recorded at the time of an action or operation. The system allows real-time data entry from any device, including mobile devices, ensuring seamless data storage, processing, synchronization, and reporting.
- Original: The system always retains the original record or document. The audit trail ensures that users always access the most current and authentic document, with electronic signature verification to confirm data integrity.
Accurate: Data must be error-free and protected against undocumented modifications. The system includes data validation procedures, type verification, and structured dropdown lists for data entry. Version control mechanisms track changes, archive document versions, and enforce the approval of new document revisions.
Key issues addressed by the EDMS
- Development of internal policies and instructions for working with electronic documents
- Registration and record-keeping procedures for electronic documents
- Implementation of electronic signature practices for personnel
- Access control for structural divisions and employees
- Monitoring document validity periods and managing amendments
- Automated electronic distribution of documents
- Controlled printing of document copies
- Tracking and removing obsolete document copies from departments
- Preparation and digitization of paper documents
- Establishing document storage policies and archival procedures
- The eGMP/GDP EDMS ensures full compliance with Ukrainian regulations and international GxP standards, providing a secure, transparent, and efficient document management system for pharmaceutical enterprises.
User support and technical assistance
We are ready to help you integrate the innovative eGMP/GDP EDMS into your business processes!
With years of experience in IT and the pharmaceutical industry, deep knowledge of Good Practice (GxP) requirements, and a commitment to continuous platform improvement, we are a trusted partner for every client. We offer flexible system configurations with multiple levels of customization, allowing you to adapt the platform to your specific needs.
We understand the importance of cost-effectiveness and strive to deliver an affordable solution without compromising quality. The eGMP/GDP EDMS is a reliable tool that enables you to efficiently manage your pharmaceutical quality system, ensure compliance with international standards, and streamline all processes.
Contact us today for a consultation or a live demonstration of the platform’s capabilities!