eCTD RIMS – dossier filling, submission and management
The system complies with the requirements of the ICH electronic Common Technical Document (eCTD) v3.2.2, and the specification of Module 1 aligns with the requirements of the Order of the Ministry of Health of Ukraine No. 691 dated April 23, 2024, including all amendments.

The electronic Common Technical Document (eCTD) is an electronic format of the Common Technical Document (CTD), developed according to an international standard by the International Council for Harmonisation (ICH). It serves as an interface for applicants to submit regulatory information to the State Expert Center of the Ministry of Health of Ukraine. The eCTD format enables the creation, review, tracking, and version management of documents, as well as the electronic archiving of registration dossier materials.
Transition to eCTD format in three phases:
- May 1 – December 31, 2024: A pilot phase for submitting registration dossier materials for marketing authorization, re-registration, and variations in the eCTD format.
- January 1 – August 18, 2025: Applicants may choose between paper-based and electronic (eCTD) submission for registration materials.
- From August 18, 2025: The eCTD format becomes mandatory, as stipulated in Clause 6, Article 13 of the Law of Ukraine “On Medicinal Products” (2022 edition).
The Order of the Ministry of Health of Ukraine No. 691, dated April 23, 2024, approves the Module 1 specification and validation criteria for Ukraine. Additionally, the Unified Information Automated System has been updated to support eCTD workflows, including the applicant’s portal, pharmacovigilance solutions, and electronic archive.
Key Advantages of eCTD RIMS by iRIS Soft:
The eCTD RIMS system not only ensures compliance with Ukrainian regulatory requirements but also meets global standards, enhancing the efficiency of the dossier submission process. Our system incorporates all essential components in accordance with best international practices.
Key features of our product:
TheeCTD RIMS system consists of several integrated modules designed to streamline the registration dossier submission process:
- eCTD Manager – Dossier creation and compilation
- eCTD Sign – Digital signature application
- eCTD Validator – Dossier validation before submission
- eCTD Archive – Secure digital archive for documents
- eCTD Codifier – Document classification and coding
- eCTD Product – Product information and dossier tracking
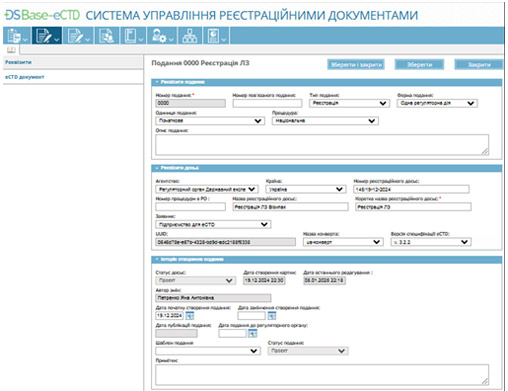
The eCTD Manager module facilitates the creation of an electronic Common Technical Document (eCTD) and provides users with:
- The ability to structure the eCTD dossier, including Module 1UA (Ukrainian-specific) and Modules 2-5 by ICH eCTD standards.
- A user-friendly, intuitive tree-view interface for easy navigation and document organization.
- Tools for envelope creation to support submission to the State Expert Center of the Ministry of Health of Ukraine (SEC MoH).
- Electronic logs for tracking and managing regulatory authority comments and responses.
- File import tools for seamless document upload and integration.
- The ability to create additional branches within the eCTD structure as needed.
- Dossier metadata management to maintain accurate registration dossier records.
- Integration with the DSBase database, enabling synchronization with the company’s medicinal product registry.

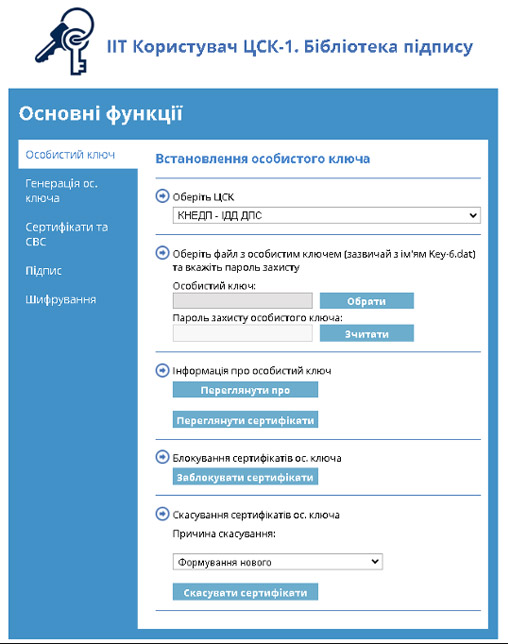
The eCTD Sign module provides software tools for digitally signing documents using a Qualified Electronic Signature (QES) in compliance with Ukrainian regulatory requirements and certified by the State Service of Special Communications and Information Protection of Ukraine (DSSZZI).
Key Features:
- The QES is stored on a secure hardware device (USB token), ensuring key security and controlled access.
- The system automatically validates the authenticity of the signature through the Key Certification Center (KCC).
User software requirements: to use QES, the “IIT User KCC-1” software package must be installed on the user’s computer. This specialized software library is supplied with the eCTD RIMS system by iRIS Soft. It ensures seamless integration with electronic signatures and guarantees compliance with Ukrainian regulations.

The eCTD Validator module performs comprehensive validation of the prepared submission before it is sent to the regulatory authority, ensuring compliance with Ukrainian and international eCTD requirements:
- Ensures the correct structure of the XML submission file.
- Verifies that eCTD document folders are named correctly.
- Confirms that files have appropriate and compliant names.
- Checks if files meet the required format specifications.
- Ensures there are no empty folders in the submission.
- Verifies that file names do not exceed allowed character limits.
- Confirms that the submission includes the required cover letter.
- Ensures adherence to other regulatory validation requirements.

The eCTD Codifier module simplifies and accelerates the transition from any format (including paper-based) to eCTD format, ensuring a seamless regulatory submission process:
- Fast reformatting: automatically converts existing dossiers to meet eCTD requirements.
- Convenience & accuracy: supports precise data migration with minimal risk of errors.
- Time optimization: reduces document preparation time for submission.
The use of the baseline submission (0000) and the DSBase-eCTD Codifier tool enables pharmaceutical companies to quickly adapt to modern regulatory requirements, ensuring transparency, accuracy, and compliance with eCTD standards.
The modern eCTD format significantly increases the volume of digital documents handled by pharmaceutical companies. To efficiently manage and process this growing amount of data, a robust digital archiving system is essential.
eCTD Archive is an advanced digital archive that provides:
- Document storage & organization. All digital files are structured and easily accessible in an organized format.
- Version control & change tracking. The system ensures transparency and accuracy by tracking document versions and updates.
- Workflow optimization. Automated document preparation and processing significantly enhances team productivity.
- Regulatory compliance. Helps maintain compliance with current regulatory standards and requirements.
- Data security & risk reduction. A reliable storage system minimizes the risk of data loss or unauthorized access.
- Collaborative work. Enables efficient cross-departmental collaboration, improving task coordination.
- Fast information retrieval. Advanced search features allow users to quickly locate relevant documents and data.
By implementing DSBase-eCTD Archive, companies can optimize document management, streamline information flows, enhance team collaboration, and ensure full regulatory compliance.