DSBase – pharmacovigilance platform

DSBase automates the recording, analysis, and assessment of adverse reactions and lack of drug efficacy, creating a reliable electronic documentation system. This system helps control, record, and manage all aspects of pharmacovigilance that may directly or indirectly impact product quality. It is an integral part of the quality assurance system and plays a key role in pharmaceutical companies, ensuring compliance with international quality and safety standards.
For over 10 years, the computerized DSBase system has been assisting pharmaceutical companies in meeting pharmacovigilance and quality requirements in Ukraine, as well as in European and Asian countries. As of the end of 2024, DSBase is successfully used by 25 companies across Ukraine, Switzerland, India, Kazakhstan, Uzbekistan, Moldova, and other countries. It is a reliable solution for risk management, documentation, and quality assurance that fully complies with pharmacovigilance requirements.
The implementation of DSBase aims to enhance a company’s pharmacovigilance system and support timely decision-making to improve drug safety. Through automation and analytical capabilities, the system enables rapid response to identified risks and facilitates well-informed decisions to protect patients.
Key functional capabilities of DSBase:
The system provides a comprehensive set of functions to support all aspects of pharmacovigilance in pharmaceutical companies, local offices and international subsidiaries, consulting firms, and independent consultants. Its modular structure enables the automation of safety monitoring processes, document management, personnel training, and quality control, adapting to the specific needs of each user. The system maintains structured pharmacovigilance data, ensuring internal processes remain compliant with regulatory requirements.
Technological features
The DSBase computerized system is designed with a focus on flexibility, scalability, and security, allowing for the efficient support and automation of complex data processing tasks. The system’s key technological advantages include:
Modular architecture: the system is built on a modular framework that allows for customization and functional expansion based on each client’s specific requirements. This flexibility makes it adaptable for both small businesses and large corporations, ensuring optimal usability.
Scalability and performance: DSBase supports high-performance operations and can process large volumes of data. This ensures the efficient management of complex business processes while maintaining system stability even under heavy workloads.
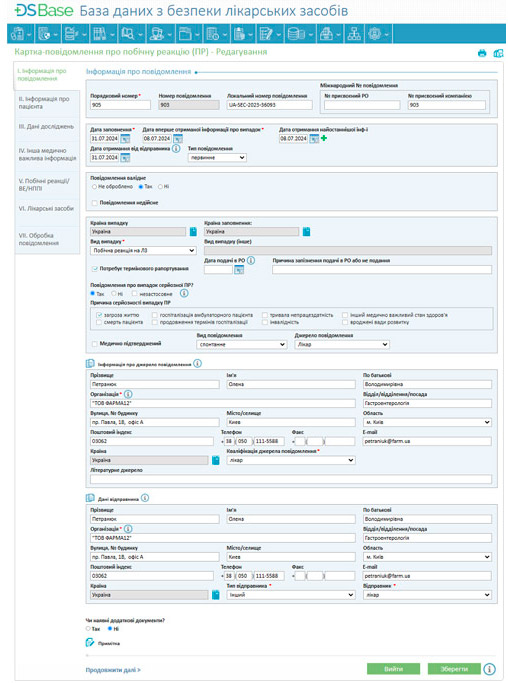
Adverse reaction data collection and processing: DSBase enables easy registration of adverse reaction cases, capturing comprehensive details about the patient, reaction description, drug, dosage, and conditions. It also facilitates follow-up communication with users submitting adverse reaction reports to clarify case details, aiding in the rapid identification of potential risks.
E2B R2/R3 compatibility: DSBase allows for the automatic upload of adverse reaction and lack of efficacy reports in E2B R2 and E2B R3 formats. It supports data export via API, ensuring fast and accurate data import while verifying compliance with standards and automatically updating information within the system. This streamlines data exchange with regulatory authorities, reducing processing time and increasing operational efficiency.
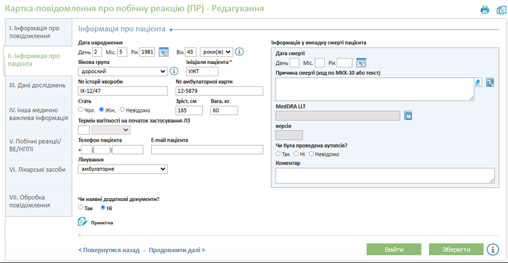
Drug registry management: DSBase includes a module for maintaining a comprehensive drug registry and product passports, containing registration details, post-market status, usage instructions, safety reports, and adverse reaction notifications. This ensures systematic storage and easy access to all critical data in one centralized location.
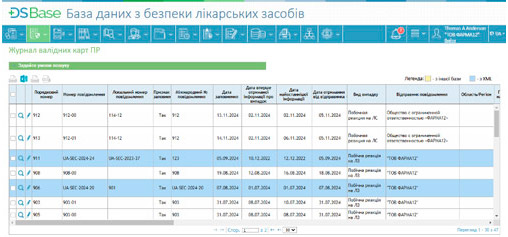
Signal detection: The system features tools for automatically generating and processing safety signals, identifying potential risks associated with medicinal products. These functionalities support a proactive risk management approach, ensuring effective drug safety monitoring.
Integration with MedDRA dictionary: DSBase supports the upload and update of the Medical Dictionary for Regulatory Activities (MedDRA), which is used for coding and classifying adverse events and reactions. This ensures accuracy in adverse reaction data processing and facilitates seamless information exchange between pharmaceutical companies and regulatory authorities.
Local literature screening: The literature monitoring module enables the tracking of drug safety information at both local and international levels, ensuring data relevance and compliance with current safety regulations.

Training and personnel testing: to maintain a high level of competency among professionals, DSBase includes a training management module, allowing for the creation of tests and monitoring of staff knowledge, ensuring continuous professional development.
Quality management: the quality management module enables the analysis of complaints, defect control, auditing, and report generation, ensuring compliance with regulatory requirements and improving overall product quality and safety.

Compliance with standards and certification
The DSBase computerized system complies with key international and national standards and regulatory requirements in the field of pharmacovigilance. This ensures high reliability in the processes of collecting, processing, and reporting drug safety data, as well as guaranteeing compliance with the regulatory requirements of different countries.
Key standards and directives complied with by DSBase:
ICH Harmonised Tripartite Guideline (ICH Directive) – DSBase adheres to the ICH guidelines, which regulate international harmonization in the management of drug safety data in clinical practice. This ensures a high level of pharmacovigilance process alignment across different markets.
ICH E2B(R2) (Data Elements for Transmission of Individual Case Safety Reports, ICSR) – The system fully supports ICH E2B(R2) requirements regarding the structure and content of individual case safety reports, facilitating seamless data exchange between pharmaceutical companies and regulatory authorities.
ICH M2 EWG (Electronic Transmission of Individual Case Safety Reports, E2B(R2) Message Specification) – DSBase implements electronic transmission of safety case reports in accordance with ICH M2 EWG specifications, ensuring efficient and secure digital information exchange.
Good Pharmacovigilance Practices (GVP) – DSBase is fully compliant with GVP guidelines, ensuring a structured and transparent process for monitoring, processing, and reporting adverse reactions in line with EU requirements.
ISO 9001 & ISO/IEC 27001 – The system meets the ISO 9001 quality management standard and ISO/IEC 27001 information security standard, ensuring reliable data storage, protection of information assets, and compliance with modern quality management requirements.
Compliance with FDA & EMA Requirements – Thanks to its compliance with FDA (USA) and EMA (EU) regulatory standards, DSBase can be used for data processing and reporting in accordance with the regulations of these authorities, making it an effective solution for companies operating in the global market.
Certification and data protection
The software solutions developed by IRIS provide robust information security through an advanced access management system, user permission control, logging, and auditing of user actions. All data and documents are protected against unauthorized interference, with security standards that meet modern information security requirements.
A centralized data and document repository ensures users have controlled and secure access to information that is both fast and protected. The unified database management system and automated backup service guarantee the reliability of document storage, eliminating the need for users to create personal copies of confidential information.
User support and technical assistance
We are ready to help you seamlessly integrate the innovative DSBase solution into your business processes. With our extensive experience in IT and the pharmaceutical industry, deep knowledge of pharmacovigilance standards, and ongoing platform enhancements, we are a trusted partner for every client.
We offer flexible DSBase customization with multiple modification levels, allowing you to tailor the platform to your individual requirements.
We understand the importance of cost-effectiveness and high-quality standards, which is why we strive to provide an affordable solution without compromising on quality. DSBase is a reliable tool that enables efficient drug safety management, compliance with international standards, and streamlined pharmacovigilance processes.



